
Not actual
patients.
CRYSVITA achieved significantly greater improvement in XLH-related rickets severity vs. conventional therapy at Week 40 in an open-labelled study (Mean RGI-C global scores [95% CI]: 1.9 [1.70, 2.14] vs. 0.8 [0.56, 0.99]; p<0.0001)1,2†‡
Mean RGI-C global scores in children 1–12 years old1,2§
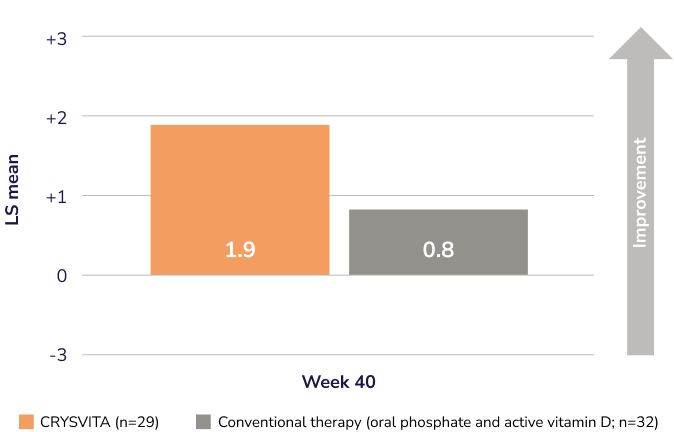
CRYSVITA (n=29)
Conventional therapy (oral phosphate and active vitamin D; n=32)
Adapted from the Product Monograph and Imel EA, et al., 2019.1,2
At Week 64, the LS mean RGI-C Global score was +2.1 in the CRYSVITA group and +1.0 in the active control group (secondary endpoint)2
Proportion of patients who achieved substantial healing of rickets (defined as RGI-C score of ≥+2.0) at Week 40 (secondary endpoint)1,2†§
of patients receiving CRYSVITA
(21/29)
VS.
of patients receiving conventional therapy
(oral phosphate and active vitamin D)
(2/32)
These findings were maintained at Week 64.1,2
Demonstrated Thacher rickets severity score (RSS) results with CRYSVITA and conventional therapy at Week 40 (secondary endpoint)1,2†
LS mean change in Thacher RSS from baseline to Week 40
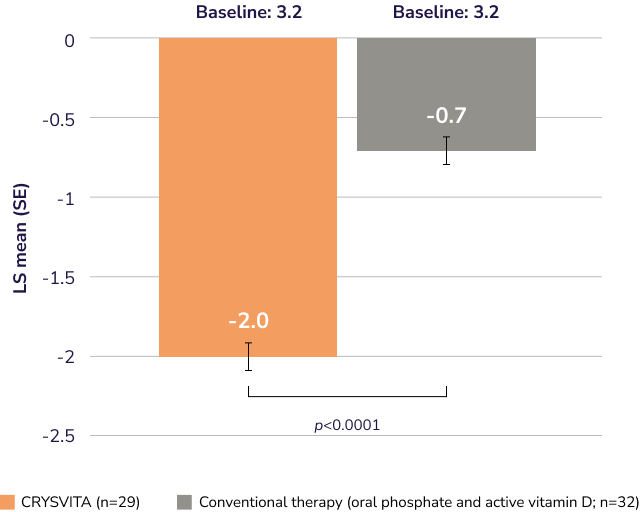
CRYSVITA (n=29)
Conventional therapy (oral phosphate and active vitamin D; n=32)
RSS data were not controlled for type I error.
Adapted from Imel EA, et al., 2019.2 ANCOVA was used for RSS, using treatment group and baseline age stratification factors as independent variables and baseline total RSS as a continuous covariate.
RSS data were not controlled for type I error.
Lower extremity skeletal abnormalities with CRYSVITA and conventional therapy at Week 64 (secondary endpoint)1,2†
LS mean RGI-C in standing long leg radiographs in children 1–12 years old1,2
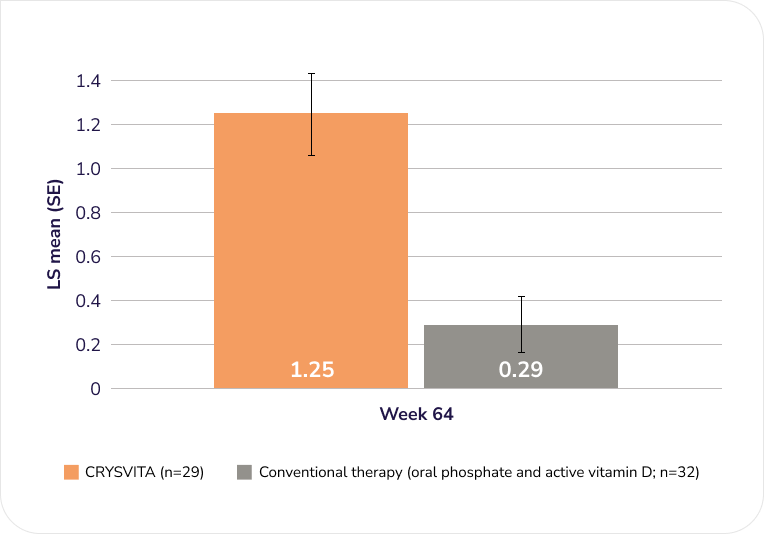
CRYSVITA (n=29)
Conventional therapy (oral phosphate and active vitamin D; n=32)
Lower extremity skeletal abnormalities were assessed by RGI-C (adapted to assess leg bowing and knock knees) in standing long leg radiographs.1,2
Adapted from the Product Monograph and Imel EA, et al., 2019.1,2
Lower extremity skeletal abnormalities were assessed by RGI-C (adapted to assess leg bowing and knock knees) in standing long leg radiographs.1,2
Demonstrated growth (height Z score) results with CRYSVITA compared to conventional therapy at Week 64 (secondary endpoint)1,2†
Increased standing mean height Z score at Week 64
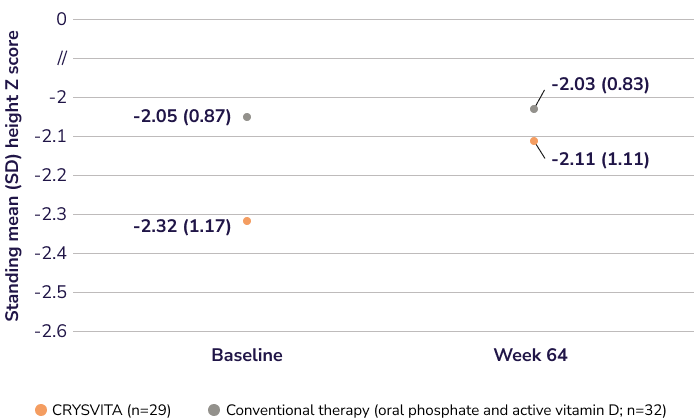
CRYSVITA (n=29)
Conventional therapy (oral phosphate and active vitamin D; n=32)
Adapted from the Product Monograph.1
Demonstrated serum phosphorus results with CRYSVITA compared to conventional therapy at Week 40 and 64 (secondary endpoint)1,2†
Serum phosphorus in children
1–12 years old1,2
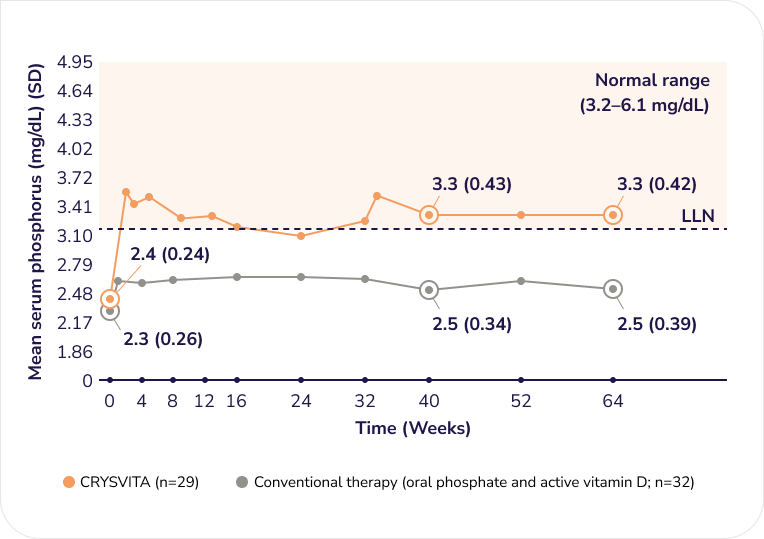
CRYSVITA (n=29)
Conventional therapy (oral phosphate and active vitamin D; n=32)
Adapted from the Product Monograph and Imel EA, et al., 2019.1,2
CRYSVITA treatment resulted in mean serum phosphorus levels that were within the normal range for children (3.2–6.1 mg/dL) at efficacy assessments at Week 40 and 64.1
Note that 1 mg/dL is equivalent to 0.323 mmol/L.3
ANCOVA=analysis of covariance; CI=confidence interval; LLN=lower limit of normal; LS=least squares; RGI-C=Radiographic Global Impression of Change; RSS=Rickets Severity Score; SD=standard deviation; SE=standard error.
† Randomized, Phase 3, active-controlled, open-label study in 61 pediatric patients (aged 1 to 12) with XLH and radiographic evidence of rickets at baseline, who had RSS score of ≥2.0 and had received oral phosphate and active vitamin D analogues for a mean (SD) duration of 4 (3.1) years. Patients were randomized to receive either subcutaneous burosumab starting at 0.8 mg/kg (n=29) every 2 weeks (the CRYSVITA group) or oral phosphate (recommended dose 20–60 mg/kg/day) and active vitamin D analogues (recommended doses calcitriol 20–30 ng/kg/day or alfacalcidol 40–60 ng/kg/day) (n=32), as prescribed by investigators (the conventional therapy group). Oral phosphate and active vitamin D analogues were discontinued prior to study enrolment for a 7-day washout period and then reinitiated for patients in the conventional therapy group. All patients completed at least 64 weeks on study. The primary endpoint was change in rickets severity at Week 40, assessed by the RGI-C global score.1,6
‡ Estimates of LS mean and 95% Cl for Week 40 are from an ANCOVA model accounting for treatment group, baseline RSS and baseline age stratification factor. Subsequent p values are based on this comparison between treatment groups.1,6
§ The RGI-C score is assigned based on side-by-side comparisons of wrist and knee radiographs from two timepoints, with higher scores indicating greater improvement in rickets.
References: 1. CRYSVITA (burosumab injection) Product Monograph. Kyowa Kirin Inc. August 13, 2025. 2. Imel EA, et al. Burosumab versus continuation of conventional therapy in children with X-linked hypophosphataemia: A randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416–2427. 3. ENDMEMO. Available from: http://www.endmemo.com/medical/unitconvert/Phosphorus.php. Consulted October 3, 2023.